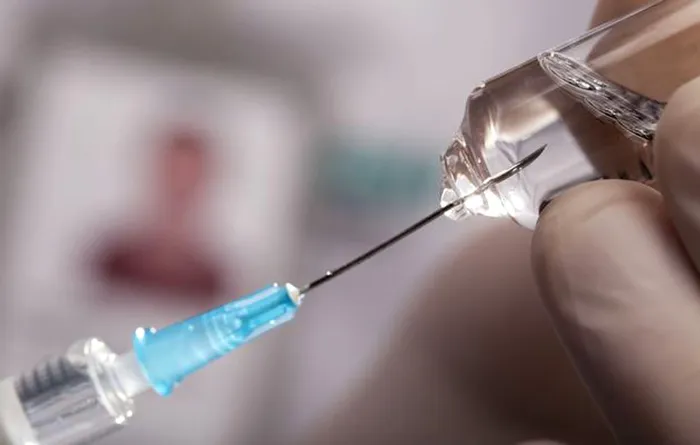
The South African Health Products Regulatory Authority (SAHPRA) has approved Lenacapavir, a six-monthly PrEP injection to prevent HIV-1 in adults and adolescents weighing at least 35 kg. CEO Dr Boitumelo Semete-Makokotlela said the approval is “a game-changer.”
Image: Sigciniwe
The South African Health Products Regulatory Authority (SAHPRA) has approved Lenacapavir, a new antiviral medicine for pre-exposure prophylaxis (PrEP) to prevent HIV-1 infection.
The drug is recommended for adults and adolescents weighing at least 35 kg.
SAHPRA said the application for Lenacapavir was submitted by Gilead in March 2025 and reviewed through the European Medicines for All Procedure (EU-M4all).
The authority said the procedure “enables the European Medicines Agency (EMA), together with the participating regulatory authorities, to provide scientific opinions on high-priority medicines, such as Lenacapavir, intended for markets outside the European Union.”
It added that the pathway strengthens regulatory systems and accelerates access to essential medicines.
Lenacapavir is administered as a six-monthly injection, SAHPRA said, starting with a subcutaneous initiation dose, supported by tablets on days one and two.
The authority said it is intended for HIV-negative adults and adolescents at risk of infection and weighing at least 35 kg.
SAHPRA emphasised that the medicine should always be used alongside safer sex practices, such as condom use, to reduce the risk of other sexually transmitted infections.
Dr Boitumelo Semete-Makokotlela, CEO of SAHPRA, said the approval of Lenacapavir is “a game-changer, given the high prevalence rate of HIV in South Africa.”
She added that the product is “the most effective HIV prevention measure thus far.”
Briefing the media week ago, Minister in the Presidency, Khumbudzo Ntshavheni, said Cabinet has welcomed the approval of Lenacapavir, highlighting the importance of ensuring uninterrupted HIV prevention services.
She said the initial rollout is scheduled for March or April 2026, targeting 23 high-incidence districts across six provinces and around 360 high-performing public clinics.
Ntshavheni added that the programme forms part of broader HIV prevention efforts, supported by the US President’s Emergency Plan for AIDS Relief (PEPFAR) Bridge Plan, which aims to strengthen HIV service delivery in South Africa.
hope.ntanzi@iol.co.za
IOL News
Related Topics: